By Mr. Marc Planchette
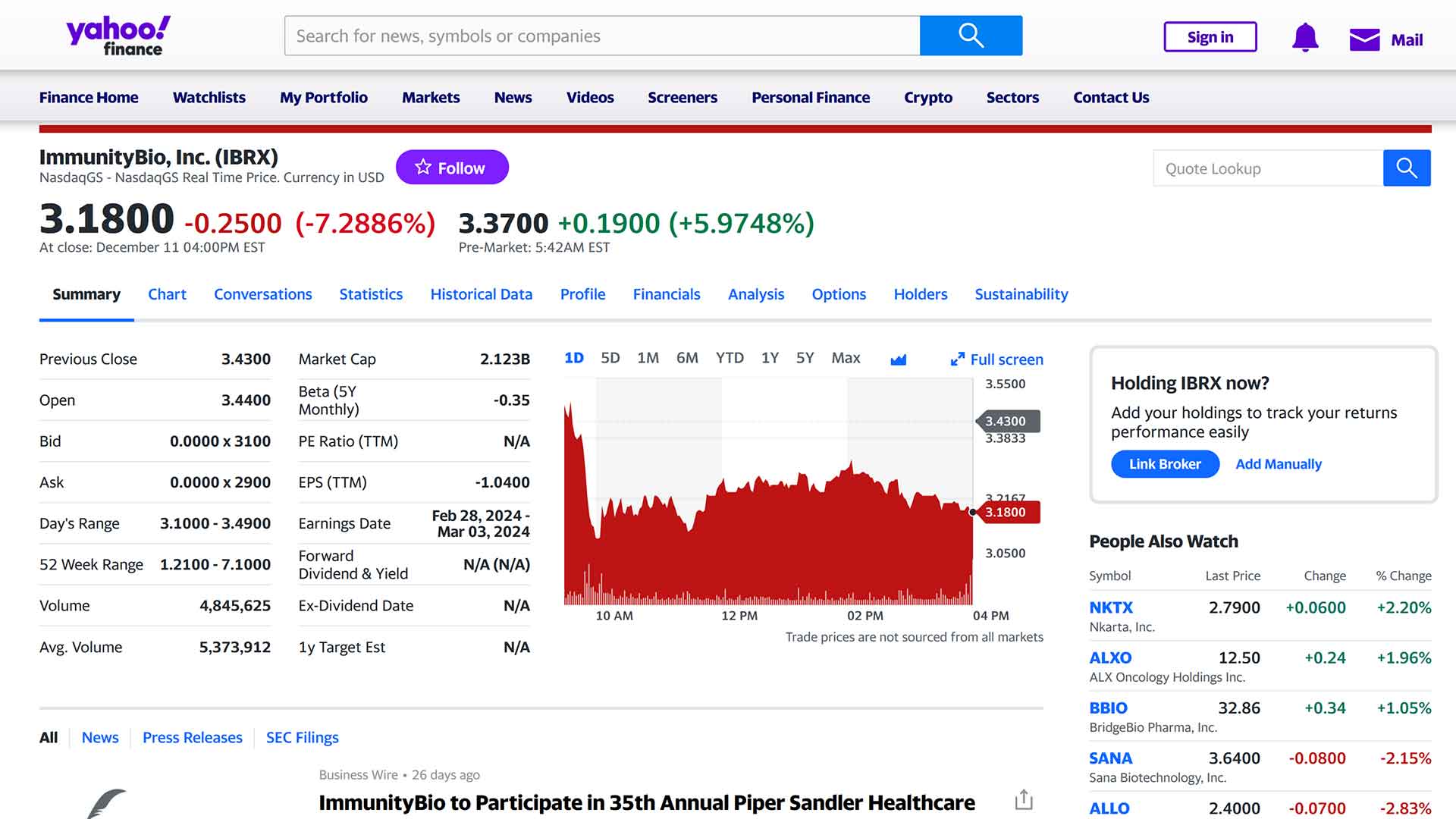
Over the last 5 years, IBRX shares successively went from $7 to $1, then rose to more than $40 during a trading session before falling back to $1.30 a few months ago, after a fall over two years. Recently, after the second submission of an application for approval to the FDA (refused during the 1st review due to manufacturing logistics issues) for their first therapy against a specific type of cancer representing a market of $20 billion, the action rose in less than a month from $1.30 to $4.40 peak during one session. Since then, sales have been made, and before the market opened, the stock was at $3.18. I estimate its potential to be around $10 by April 23, 2024, the date on which the FDA could approve this first therapy.
Immunotherapy clinical portfolio
ImmunityBio has an extensive immunotherapy clinical portfolio of more than 40 clinical trials in Phase I, II and III development across 19 indications in solid and liquid cancers and infectious diseases. The clinical-stage portfolio and intellectual property portfolio encompasses 17 first-in-class human antibody cytokine fusion proteins, chemo-modulators, vaccine vectors and cell-based therapies in 25 Phase II and III clinical trials.
Anktiva™ (ImmunityBio's lead cytokine infusion protein) is a novel interleukin-15 (IL-15) superagonist complex and has received Breakthrough Therapy and Fast Track designations from the Food and Drug Administration (FDA) of the United States for CIS non-muscle-invasive BCG-insensitive bladder cancer (NMIBC).
Board of Directors
ImmunityBio's nine-member board of directors is led by founder and executive chairman Patrick Soon-Shiong, MD. The board includes two other recently appointed external members, former CIA director John Brennan and Gen. of retired U.S. Army Wesley Clark, as well as current board members Michael Blaszyk, Cheryl Cohen, Christobel Selecky and Barry Simon, MD. ImmunityBio recently appointed Dr. Linda Maxwell and CEO Richard Adcock to the board of directors.
About ImmunityBio
ImmunityBio, Inc., a clinical-stage biotechnology company, engages in the development of therapies and vaccines that complement, harness and amplify the immune system to defeat cancers and infectious diseases in the United States and Europe. It offers immunotherapy and cellular therapy platforms, including antibody-cytokine fusion protein N-803, vaccine technologies, Toll-Like receptor activating adjuvants, natural killer cells and damage-associated molecular pattern inducers . The company is also developing therapeutic agents, which are in phase II or III clinical trials for the treatment of liquid and solid tumors, including bladder, pancreatic and lung cancers, as well as pathogens such as SARS -CoV-2 and HIV.
Collaboration agreements
It has collaborative agreements with the National Cancer Institute and Amyris, Inc.; and licensing agreements with LadRx Corporation, GlobeImmune, Inc., Access to Advanced Health Institute, 3M Innovative Properties Company, Sanford Health, Shenzhen Beike Biotechnology Co. Ltd., Sorrento Therapeutics, Inc. and Viracta Therapeutics, Inc. The Company is based in San Diego, California.
WARNING - Significant risk of loss on the stock market
The risk of loss on the stock market and in particular on futures and/or options contracts is significant and each investor and/or trader must independently determine whether it is a suitable investment. Past performance, whether actual or indicated by simulated historical testing of strategies, is not indicative of future results. This communication is not a solicitation.
*I am not an advisor and this investment idea is not a buy or sell recommendation. Ask your advisor before buying or selling.*
 e095d795-98fe-11ee-b11c-a0423f4b9730
e095d795-98fe-11ee-b11c-a0423f4b9730